Chemical Topics
The chemical topics officially finished the first module! Professor Abrahams finished the topic by discussing a variety of examples in supramoleculer and inorganic chemistry. I thought the discussion on catenanes and rotaxanes was particularly interesting because these molecules are "interlocked". Catenanes have interlocked macrocycles (rings), while rotaxanes have an interlocked stopped by two "stoppers". You can see and example of this below!
 |
| Catenane (Wikipedia) |
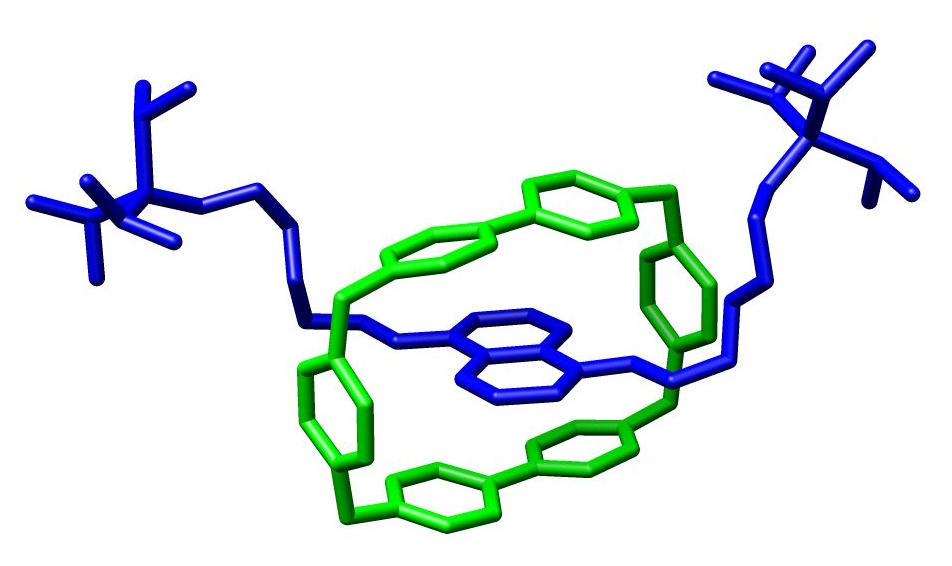 |
| Rotaxane (Wikipedia) |
The second module, Computational Chemistry, started this past week. Instead of having a lecture and a tutorial, we only have a lecture for this module because it is run in a more tutorial/workshop style. There are three different lecturer's for this topic.The first lecturer, Professor Richard O'Hair prepared exercises on solvation and organometallic reactions (I'll talk about this later). First, we calculated the conformational energies (ie the energy for a specific geometry of the molecule) of the amino acid glycine (L), preferred in the gas phase, and its zwitterion form (R), which is preferred in solution using Hartree Fock methods. We then did the same thing taking into account water as a solvent. Everyone in the class did this: drew the molecule and ran code to obtain the energies. One of the questions were are looking to answer is why everyone had slightly different energies! However, this can be explained by geometry. For a single molecule, take the netural glycine on the left for example, it is possible for atoms to rotate around bonds and certain orientations are more favorable than others. So one student may have drawn a less favorable geometry than another, accounting for the slight energy differences.
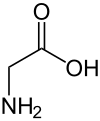 |
| Neutral Glycine |
 |
| Zwitterion |
Research Project
When I don't have class I go into lab, because the number of days we have before we have to submit reports seems to me to be diminishing quickly! I have been work on setting up LOTS and LOTS of crystallizations. In essense reacting a copper source (like copper acetate, copper nitrate, copper sulfate) with our ligand and different combinations of other counter ions. I just set up a huge batch before this weekend, so hopefully I'll get some really nice crystals to go over for Monday morning. I'm not exactly sure when this is going to happen, but Tim, the postdoc who supervises me, said that he would try to get us time on the diffractometer next week!
Anthropology and Australia Now
I had papers due for both of these classes this week! It was actually really difficult to figure out what to write about, but for my anthropology class I ended up discussing the theorist Mary Douglas and her interpretation on religious food prohibition. Meanwhile, in Australia Now, I chose to reflect on two topics we covered in class, multiculturalism/immigration and Indigenous history.
Best,
Stephanie
No comments:
Post a Comment